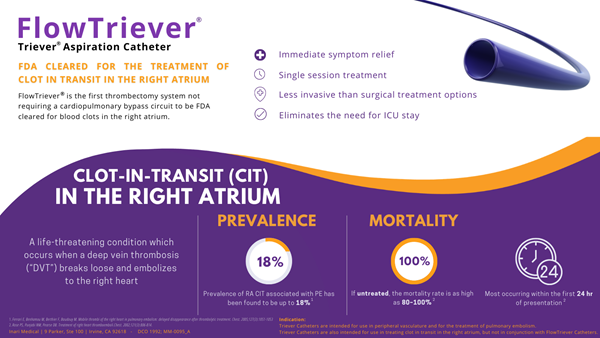
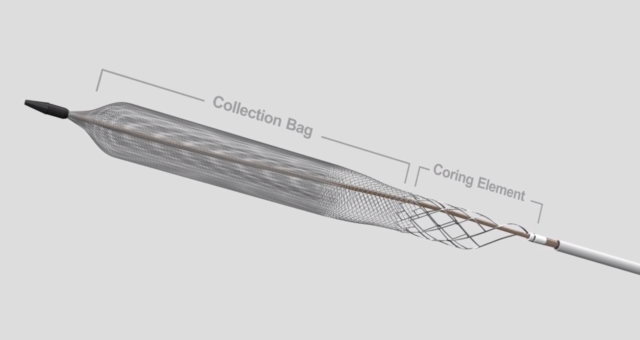
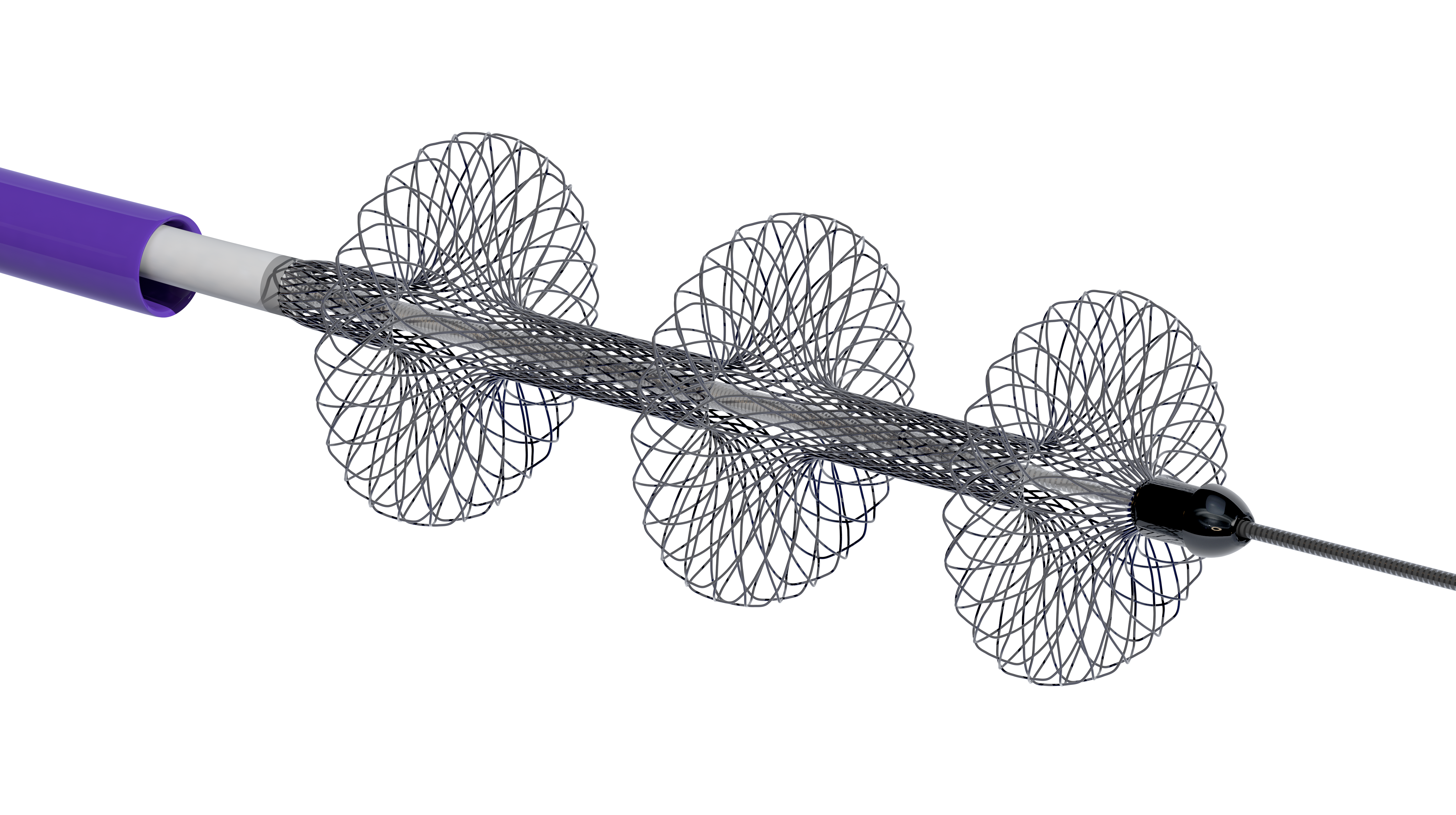
Inari Medical has a unique story. In 2013, Inari Medical spun-out of the incubator Inceptus Medical after it developed two thrombectomy technology platforms for the treatment of peripheral vessels. These technologies were financially backed by prestigious MedTech venture capital firms, Gilde Healthcare, Versant Ventures, and U.S. Venture Partners. Both technologies have FDA clearance, and in 2019 Inari Medical made history by being the most profitable MedTech startup in the first year of sales with $51 million in revenue. After going public in May, Inari Medical was named the best IPO debut of 2020. Oh, and Inari Medical is named after a piece of sushi…inari sushi.
We have partnered with Ray Su, Head of Biostats, in hiring a Biostatistician for Inari Medical. This newly created role will be responsible for statistical design and analysis of clinical research studies as well as for providing statistical expertise for publication efforts and regulatory submissions.